Halogen Bonding
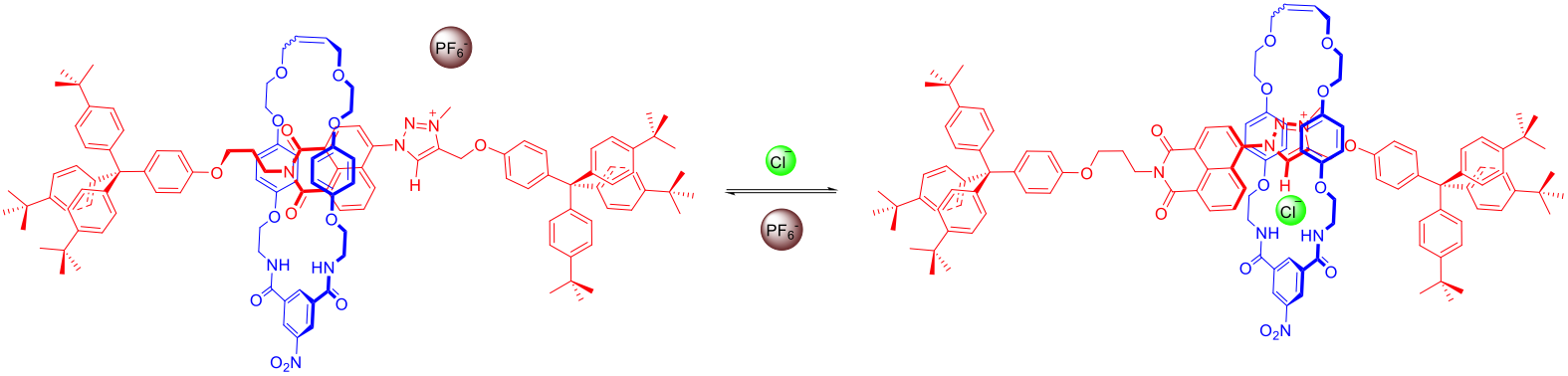
Halogen bonding (XB) is the non-covalent bonding interaction between halogen atoms which function as electrophilic centres (Lewis acids) and neutral or anionic Lewis bases. Of the many non-covalent interactions commonly utilized in solution phase supramolecular assemblies, halogen bonding is arguably the least exploited, which is surprising given its potentially powerful analogy to ubiquitous hydrogen bonding (HB). We have reported the first examples of solution phase halogen bonding being exploited to recognize and sense anions in aqueous media, and to control and facilitate the anion templated assembly of rotaxane and catenane interlocked structures (see figure below), including a molecular shuttle.
Importantly, the incorporation of halogen bond donors into acyclic, macrocyclic, and interlocked host cavities dramatically enhances anion recognition compared to hydrogen bond donor host analogues in aqueous media. For instance, we recently demonstrated the exploitation and quantification of XB in water for the first time using water-soluble acyclic and rotaxane hosts prepared from mono-functionalised permethylated β-cyclodextrin derivatives. Incorporation of XB-donors into such receptors resulted in a dramatic enhancement of anion binding in water compared to the equivalent HB analogues, with up to two orders of magnitude enhancement observed in the interlocked XB rotaxane host system (see figure below).
In collaboration with Professor Pierre Kennepohl (University of British Columbia), we have also investigated the nature of halogen bonds through X-ray Absorption Spectroscopy (XAS): these studies revealed charge transfer from the halide to its halogen bonding partner, and demonstrated that the degree of covalency is similar to that which is observed in transition metal coordinate covalent bonds.
These results highlight the superiority of XB as an intermolecular interaction for anion binding in water, and exemplify the exciting potential of XB in other applications requiring molecular assembly in aqueous environments that have been traditionally dominated by the ubiquitous hydrogen bond, including self-assembly, green-chemistry, catalysis and drug discovery.
Selected References
"Evidence for Halogen Bond Covalency in Acyclic and Interlocked Halogen-Bonding Receptor Anion Recognition"
S. W. Robinson, C. L. Mustoe, N. G. White, A. Brown, A. Thompson, P. Kennepohl, P. D. Beer.
J. Am. Chem. Soc. 2015, 137(1), 499-507.
"Halogen bonding in water results in enhanced anion recognition in acyclic and rotaxane hosts"
M. J. Langton, S. W. Robinson, I. Marques, V. Felix and P. D. Beer.
Nat.Chem. 2014, 6, 1039-1043.
Cover picture December 2014, featured in Nature Chemistry News and Views: Nat. Chem. 2014, 6, 1029-1031.
"Iodide-induced shuttling of a halogen- and hydrogen-bonding two-station rotaxane"
A. Caballero, L. Swan, F. Zapata and P. D. Beer.
Angew. Chem. Int. Ed. 2014, 53, 11854-11858.
"An all-halogen bonding rotaxane for selective sensing of halides in aqueous media"
B. R. Mullaney, A. L. Thompson and P. D. Beer.
Angew. Chem. Int. Ed. 2014, 53, 11458-11462. Selected to be featured on inside cover of journal issue.
"A catenane assembled through a single charge-assisted halogen bond"
L. C. Gilday, T. Lang, A. Caballero, P. J. Costa, V. Felix and P. D. Beer.
Angew. Chem. Int. Ed. 2013, 52, 4356-4360.
"Fluorescent charge-assisted halogen-bonding macrocyclic halo-imidazolium receptors for anion recognition and sensing in aqueous media"
F. Zapata, A. Caballero, N. G. White, T. D. W. Claridge, P. J. Costa, V. Felix and P. D. Beer.
J. Am. Chem. Soc. 2012, 134, 11533-11541.
"A halogen-bonding catenane for anion recognition and sensing"
A. Caballero, F. Zapata, N. G. White, P. J. Costa, V. Felix and P. D. Beer.
Angew. Chem. Int. Ed. 2012, 51, 1876-1880.
"A bidentate halogen-bonding bromoimidazoliophane receptor for bromide ion recognition in aqueous media"
A. Caballero, N. G. White and P. D. Beer.
Angew. Chem. Int. Ed. 2011, 50, 1845-1848.
"Enhancement of anion recognition exhibited by a halogen-bonding rotaxane host system"
N. L. Kilah, M. D. Wise, C. J. Serpell, A. L. Thompson, N. G. White, K. E. Christensen and P.D. Beer.
J. Am. Chem. Soc. 2010, 132, 11893-11895.
"Halogen Bond Anion Templated Assembly of an Imidazolium Pseudorotaxane"
C. J. Serpell, Christopher J.; N. L. Kilah, P. J. Costa, V. Felix, P. D. Beer.
Angew. Chem. Int. Ed. 2010, 49, 5322-5326.